
Emergency units outside the Puerto Rico’s Medical Center to treat people and provide COVID-19 tests. (Photo by Eric Rojas | Centro de Periodismo Investigativo)
By Luis J. Valentín Ortiz and Cristina del Mar Quiles
Versión original en español aquí.
SAN JUAN — The government of Puerto Rico’s procurement process for materials and equipment to deal with the coronavirus emergency has been plagued with mistakes, delays, inefficiency, questionable purchases and a lack of details.
The Center for Investigative Journalism (CPI, in Spanish) interviewed more than three sources who identified Adil Rosa Rivera as responsible for buying supplies and equipment for the emergency, such as ventilators, tests and protective equipment.
Up until a few days ago, Rosa Rivera coordinated the procurement process, first through the Health Department and then through the Bureau for Emergency and Disaster Management’s (NMEAD, in Spanish) Emergency Operations Center (COE). From the COE, together with Medical Task Force members Dr. Juan Salgado and Dr. Segundo Rodríguez, they have coordinated what to buy and from whom, three sources confirmed.
Rosa Rivera previously worked as the Family Department’s Administration Director for the Caguas region, and until last week was in charge of the Health Department’s Undersecretary of Administration office. She arrived at the agency under the tenure of former Health Secretary Rafael Rodríguez Mercado and his chief of staff, Mabel Cabeza Rivera.
Two sources identified Cabeza as the person La Fortaleza appointed to supervise the purchases handled by Rosa Rivera and the Task Force from the Health Department and the COE.
“I was temporarily assigned to work in La Fortaleza. There, I was asked to work with La Fortaleza’s Task Force led by Dr. Segundo Rodríguez. I wasn’t given a specific title or duties. […] I was neither a liaison nor a coordinator,” said Cabeza in a written statement. On March 30, she left Health for the executive mansion.
“I’ve never had any role in the purchasing or contracting process of the Health Department, La Fortaleza or the emergency,” she added.
But sources validated that until March 30, Cabeza was the link between the executive mansion and the Medical Task Force, and she referred names of companies to Rosa Rivera so she would ask for quotations.
Several companies referred by La Fortaleza managed to sell their products to the Government, including the three companies that provided rapid tests to the Health Department: Zogen (whose authorized representative on the island is 313 LLC); Castro Business; and Maitland 175 Inc. The process requires a review before transactions are completed by the Office of Management and Budget (OGP, in Spanish), the Treasury Department and the Health Department.
Cabeza denied giving Rosa Rivera instructions or suggestions regarding suppliers. “Any email or text message related to proposals that came to me was sent to the Health Department so they would be the ones to evaluate it and make their decisions. I have NEVER issued any recommendation to any legal person or entity regarding procurement of tests or any other acquisition that was under the Task Force’s consideration,” she said.
The sources said Rosa Rivera channeled suppliers that approached the Government and the Task Force during the emergency, asking for proposals according to the demands of the group of doctors, particularly from Dr. Salgado and Dr. Rodríguez. Although the Task Force insists that its role is limited to recommending what is needed and evaluating the available options, more than three sources said that both Dr. Rodríguez and Dr. Salgado have decision-making power and issue out instructions on procurement.
Cabeza identified Rosa Rivera, former Acting Health Secretary Dr. Concepción Quiñones de Longo and Dr. Rodríguez as the people responsible for the evaluation and procurement process. “I did not participate in the procurement and/or contracting meetings that they held,” she declared.
The CPI learned that federal authorities interviewed Rosa Rivera regarding the purchases. Rosa Rivera is a New Progressive Party (PNP, in Spanish) activist and she has publicly shown her support on social media for the party, Gov. Wanda Vázquez Garced and former Gov. Ricardo Rosselló Nevares, among other people associated with the PNP. According to a source, there is a friendship between Cabeza and Rosa Rivera, but Cabeza said it is a professional relationship.
The three secretaries the Health Department has had in the past month have appointed Rosa Rivera to oversee the agency’s procurement process. An internal agency communication dated April 3 announced the appointment of Johnny Colón González to the position of Health’s undersecretary of Administration, but it is unclear whether Rosa Rivera continues to work in the Health Department. The agency did not answer whether she was removed from her post or if she is still involved in the procurement process.
The CPI tried to contact Rosa Rivera but it was unsuccessful.
In an interview with the CPI, Dr. Salgado admitted his intervention in the procurement process from the COE and denied that he recommended companies owned by friends or acquaintances, although he did refer suppliers who have contacted him. A request for an interview made to Dr. Rodríguez went unanswered.
At least three sources said that La Fortaleza has directly intervened in the procurement process, mainly through Cabeza, when she oversaw the purchases that Rosa Rivera and her group conducted.
The Governor reiterated on Wednesday that La Fortaleza did not intervene in the process, including the order for a million rapid tests that was later canceled. She held the Health Department responsible.
The CPI asked former Acting Health Secretary Dr. Quiñones de Longo, whether the purchasing orders from the Health Department handled under her responsibility were being processed through La Fortaleza.
“That’s right, purchases and quotations were managed from La Fortaleza and they contacted us only to sign the approval. I believed the processes were inadequate, which is what ultimately motivated my resignation, because I was unwilling to participate in that kind of procurement process, because I believe they weren’t in the Health Department’s nor the Government’s best interests,” she replied.
Cabeza insisted to the CPI that “I had nothing to do with the procurement or awarding process.”
Quiñones de Longo approved the purchase of tests and other materials for the emergency carried out under her tenure. Two sources assure that the doctor evaluated the orders and had a person from the Centers for Disease Control (CDC, in English) assisting her.
The purchasing of materials and equipment was done through the Health Department until March 26. That day, NMEAD took control of the process but only on paper, according to what José Burgos, the head of the Bureau, told El Nuevo Día. Burgos identified Rosa Rivera as the person who directed the purchase of a million rapid tests which was canceled last week. Burgos did not grant a request for an interview.
Apex, the company that would supply these tests, had submitted a quotation before the COE requested it. The CPI saw evidence that the company’s proposal had been previously submitted by Juan Maldonado, former director of the Maritime Transport Authority and Apex’s lawyer, to three people: Dr. Rodríguez, Dr. Roberto Rosso —an assistant to Quiñones de Longo who resigned after she lef — and Cabeza.
The evidence also shows that Eduardo “Tito” Laureano, linked to the PNP and who worked for former Gov. Pedro Rosselló, helped establish contact between the Task Force and Apex’s attorney, Maldonado. Laureano, who Dr. Rodríguez hired at the University of Puerto Rico’s Medical Sciences Campus —as Metro reported— is Dr. Rodríguez’s friend, a source said.
At a press conference last Wednesday, Dr. Rodríguez avoided answering questions directly about his relationship with Laureano but admitted he had gotten Maldonado’s and Apex’s contact information from him.
Another source said Dr. Salgado keeps a close friendship with Maldonado’s father.
Meanwhile, Cabeza said she did not know anyone related to Apex. “I never recommended this company or any other. I never solicited a quotation from any of the companies that the Health Department or the NMEAD contracted. I didn’t recommend any of them.”
The transactions to buy tests to detect the coronavirus show the irregularities and setbacks of the Government’s procurement process to tackle the epidemic.
The Health Department showed evidence of the delivery of the first tests that arrived in Puerto Rico on March 17 —200 molecular or “PCR” tests from Quest Diagnostic— except for 50 that went to the Luis Muñoz Marín International Airport. The agency said the tests were handed over to an employee of Medical Services Administration and were intended to be used at the airport. These are the tests that Quiñones de Longo said were delivered outside the agency’s chain of command.
Cabeza stated that Rosa Rivera was the person responsible for the receipt and delivery of the tests. But a source said that Rosa Rivera did not have yet that responsibility when the tests arrived on March 17.
A request for all purchase orders issued by the Health Department and NMEAD during the emergency went unanswered.
The “Rapid” Tests
In addition to the “PCR” tests, the Government overpaid about 1.3 million serological or “rapid” tests, another type of test that detects antibodies to COVID-19. The Health Department and NMEAD handled the purchases, and when they were bought, none of the brands were backed by the Federal Food and Drug Administration (FDA). Several of the vendors have ties to the PNP.
Rosa Rivera, the Task Force and the Health Department Secretary’s office gave the go-ahead to buy the tests, three separate sources said. As for NMEAD’s, the Task Force approved the failed transaction, in the absence of a Health Secretary at the time, another source said.
On Wednesday, the Governor said it was Mariel Rivera, a Health Department mid level employee working at the COE who gave the go ahead. But Rivera has no authority to approve purchase transactions, the CPI found.
Of the total number of rapid tests that the Government bought, NMEAD acquired one million of the Promedical brand, at $38 each, for a total of $38 million—or about 200% more expensive than the market value at the time, according to sources and documents reviewed by the CPI.
After prepaying half of the invoiced amount, the Government assured it canceled the order because the company did not comply with the established delivery time and because the tests did not comply with FDA regulations, and got the money back. The acquisition was from a company named Apex, whose owner, Robert Rodríguez López, also has ties to the PNP and 313 LLC, as El Nuevo Día reported.
In a written statement, the founding partner of 313 LLC, Ricardo Vázquez Hernández, denied having a relationship with Rodríguez López and Apex.
But what the Government has not said is that the Health Department bought more than 100,000 rapid tests from 313 LLC, for which it also overpaid, just like NMEAD did for the Promedical tests. Ricardo Vázquez Hernández registered 313 LLC in Puerto Rico in 2018, and two days before the transaction, modified the incorporation certificate to include three new partners: Juan Suárez Lemus; Wilfredo Rodríguez Moreno; and Miguel García Robles, Caribbean Business reported last Saturday. Suárez Lemus and Vázquez Hernández are PNP party donors.
The CPI found that Vázquez Hernández also appears as the owner of Vertical Consulting PSC. The company is a legal advisory firm, according to the tax incentives decree that Vázquez Hernández received in 2019 from Puerto Rico Trade and Export as a young entrepreneur through a business creation initiative.
The nearly 100,000 NovaTest kits sold by 313 LLC were purchased for $45 and $26, despite the fact that there were other similar options on the market at a lower cost. The Health Department prepaid more than $2.2 million.
At a press conference, the Governor distanced herself from the procurement process. She said that people approached her and members of the Task Force to offer them rapid tests and that they referred them to the Health Department. “Quotations were requested and these people submitted them,” she said without specifying who made the first approach.
“I may not know the details, but all of this was discussed in the multiple meetings I had… The governor cannot get into micromanagement and I trust my officials,” she said to justify her lack of knowledge of the issue.
In addition, she justified the purchases at a premium by pointing to the international demand for the product, although she acknowledged that she was unaware of the profit margin the companies would generate from the transaction.
The current market price for this type of test fluctuates between $13 and $20, said Juan Rexach president, of the Puerto Rico Clinical Laboratories Association.
“I’m sure that if you buy in large quantities, you can negotiate a better price,” said the physician who holds a license in medical technology.
The two NovaTest purchase orders were never canceled, as Health Secretary Lorenzo González confirmed to the CPI on Tuesday, although it wasn’t until that same day that the test was included in the FDA’s list of brands that have been independently validated to identify COVID-19 antibodies, but that the FDA has yet to review.
In the interview, González said he was unaware of the NovaTest kits.
The Health Department’s purchase orders show 313 LLC’s Vázquez Hernández as the contact person for a company named Zogen, which he said is the authorized distributor in America for China-based Atlas Link, maker of the NovaTest kit. Zogen is a Mexican company that has Vázquez Hernández listed as its contact person in Puerto Rico.
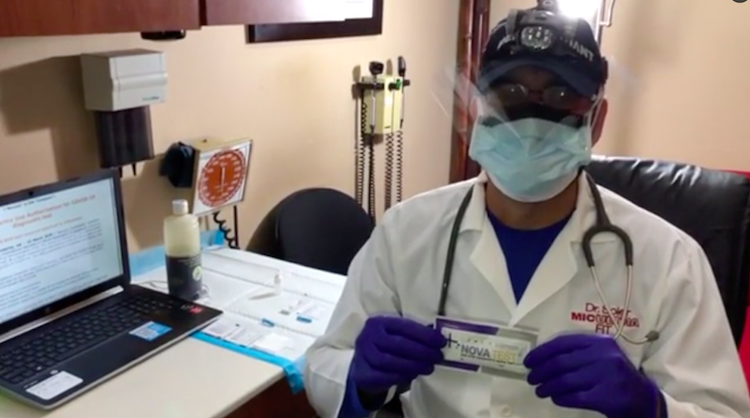

Dr. Michael Soler
Dr. Michael Soler told El Nuevo Día a week ago that the government had already received more than 1,000 NovaTest kits and that 500 more were “on their way.” The number matches the 1,500 NovaTest included in the first order made to 313 LLC. Soler added that Zogen sent two samples of its product, one to him and the other to the Health Department.
The agency used its sample during a press conference, according to Soler, who used his in this demo video for El Nuevo Día in which an FDA authorization that does not correspond to the NovaTest kit — but rather a test belonging to a company called Novacyt — can be seen on the computer screen.


The doctor did not answer a request for an interview.
The CPI found that as of March 28, the Health Department had already received and distributed almost 1,000 NovaTest kits to hospitals throughout the island, prior to getting the FDA’s approval. The agency was unable to explain the whereabouts of the NovaTest kits that have been distributed.
As of Saturday afternoon, although more than 2,000 tests had been distributed, the government had not conducted any of the rapid tests, Health Secretary González said at a press conference.
When asked for a breakdown of the rapid tests received and delivered, the Secretary said he would submit a report with the results. “It has to be somewhere in the agency.” That report has not been released to the CPI.
González reiterated that upon his arrival to his post, the procurement process changed, including requiring more than one quotation for each order, seeking the evaluation of federal agencies prior to the purchase and avoiding the use of go-betweens.
Without providing further details, González said he would refer on Tuesday everything he has uncovered related to the procurement process at the agency to the proper authorities. The Justice Department announced it launched an investigation limited to the rapid tests acquired by NMEAD from Apex. The Fiscal Control Board also asked to review the rapid test purchase orders since they were not submitted to them for an evaluation.
González told the CPI that he asked for a meeting with the Board on Tuesday to understand the process they are requiring, “recognizing that they have jurisdiction” and that he will add such requirements to the agency’s procurement structure.
Former Health Secretary Dr. Concepción Quiñones de Longo approved the more than 300,000 rapid tests acquired through the Health Department, according to two sources.
Although the Governor implied that former secretaries Rafael Rodríguez Mercado and Quiñones de Longo were solely responsible for the failed procurement of the rapid tests, the only purchase order that has been canceled to date was authorized and signed through the NMEAD and not the Health Department.
“That is where the controversy stems from, because I didn’t agree with what was being done. They treated me badly by wanting to force me to do things with which I didn’t agree,” she said, adding that she is cooperating with different ongoing investigations related to these purchases.
Overpriced Purchases and People With PNP Ties
In addition to the NovaTest kits, the Health Department purchased 50,000 rapid tests from a second vendor, Castro Business. At $13 for each test, it is the only transaction done at market value prices.
Castro Business is registered in Puerto Rico under the name of Ricardo “Ricky” Castro Ortiz, former Chamber of Marketing, Industry and Food Distribution (MIDA, in Spanish) president and also a PNP donor. In 2017, the Comptroller’s Office singled out Castro for irregularities in two multimillion dollar contracts with the National Guard. The government received and distributed several of these tests before they got FDA clearance.
The CPI tried to reach Castro Ortiz by phone but it was unsuccessful.
This outlet asked the Health Department about the status of these 50,000 tests but received no response.
In an interview with the CPI, González said: “I can tell you about last week, what we have found. We received around 7,000 tests, they came in last Friday. I don’t have details about the company. They did arrive in Puerto Rico implying that they passed through U.S. Customs. There was a concern from the FDA about the regulatory aspect.”
González explained that the FDA requested changes to the test’s labels and instructions before they could be used. He added that 200,000 additional tests arrived on Sunday and that the final delivery was completed on Monday, after getting the FDA’s approval. It remains unclear whether these are the tests the Health Department bought from Florida-based Maitland 175 Inc, with a purchase order for the same amount.
The CPI found that two of the rapid test manufacturers are Healgen, another Chinese company, and Phamatech, a California-based company. Both are listed in the FDA registry.
FDA Warns of Misinformation
Part of the delay in testing for the coronavirus in Puerto Rico is attributed to the fact that until now the island has relied on molecular tests, rather than serological, or “rapid” tests, which can take minutes to produce a result. However, the rapid test is less reliable than the molecular test since it only comes up positive if it identifies antibodies against the virus. Contrary to molecular tests, this increases the possibility of false positives or negatives in people who are asymptomatic despite having the disease.
“Serological tests will play an important role in research and surveillance but are not currently recommended for case detection,” the World Health Organization states in its Laboratory testing strategy recommendations for COVID-19.
Molecular tests from Quest and LabCorp, which provide service to municipalities and the Health Department, are on the FDA list.
The FDA’s diagnostic policy allows tests to be used without its authorization, provided they are properly labeled, including a statement that they cannot be used as the sole basis for diagnosis or to rule out infection.
The federal agency has been emphatic that the tests that have not received an authorization for emergency use, such as those bought by the Government of Puerto Rico, cannot be declared as “authorized” because “they are not.”
Given the COVID-19 emergency, the FDA has allowed the “unapproved medical products or unapproved uses of approved medical products” to be used to diagnose, treat, or prevent serious or life-threatening diseases when there are no adequate, approved, and available alternatives through an Emergency Use Authorization. Only the CDC’s, New York public laboratories, and some 28 companies have such authorization for their COVID-19 screening tests.
As the spread of infection has picked up the pace in the United States, the FDA issued on March 16 a new “Policy for Diagnostic Tests for Coronavirus Disease-2019 during the Public Health Emergency,” which states that it will not object to the development, distribution and use of serological tests to identify antibodies against SARS-CoV-2, also known as rapid tests. However, it establishes that the test must be validated by the laboratory where it will be administered and include the warning that it has not been reviewed by the FDA and that it should not be used as the only basis to diagnose or rule out infection with the virus.
The president of the Puerto Rico Clinical Laboratories Association said there is no uniform validation process for rapid tests in Puerto Rico.
The Clinical Laboratory Improvement Amendments (CLIA), which are standards that apply to clinical laboratory tests performed on humans in the United States, offer guidelines for validating tests that have not been approved by the FDA. They establish that the test to be validated must be used on among five to 10 patients who have tested positive for the molecular test (PCR), and on the same number of patients who have had a negative result.
“That way, I confirm that the tests are working, but that process must also be documented, and that process takes time,” said Rexach. So, he said that as soon a delivery of tests arrives, each hospital, clinic and laboratory that gets them has to reserve about 20 of them to complete this process before using them on the general population for which they have been designated.
An industry source said the validation process can take about three days from the time the laboratory receives the test.
Editor’s Note: Promedical has denied being contacted by Apex. It also noted that it has not done business with Apex or anyone of its contractors.
IMPORTANT development about the rapid tests controversy in #PuertoRico and price gouging by Apex. #COVID19 https://t.co/U1EpXW0w9k
— Julio Ricardo Varela (@julito77) April 14, 2020
***
Omaya Sosa Pascual and Rafelli González contributed to this story.


[…] The video shows a line of cars honking their horns. Some of the drivers held signs calling Vázquez a corrupt governor, in response a controversy involving the $38 million purchase of faulty rapid COVID-19 tests, a story that has raised serious questions about government accountability. […]
[…] The CPI revealed this week that the Health Department lacks an effective and centralized process for receiving and reporting rapid test results, which has prevented the agency from offering reliable information so far. Although thousands of rapid tests have been distributed to hospitals and health centers, the Government of Puerto Rico also had no control over what happened to these tests once they were distributed. […]